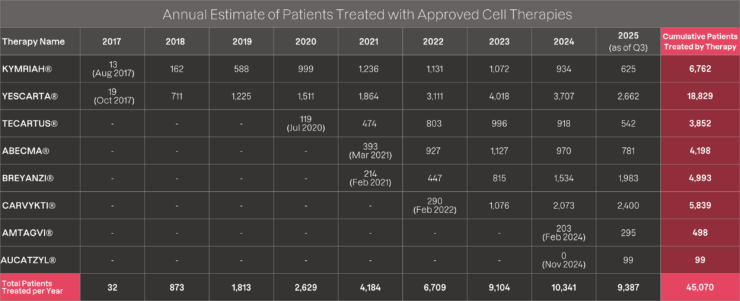
Updated Nov 2025. Note: Figures exclude Tecelra, originally developed by Adaptimmune and acquired by US World Meds in August 2025. Since its launch in August 2024, Tecelra was administered to 2 patients in 2024 and 22 patients during the first half of 2025.
To calculate the number of patients that have had the opportunity to access commercially available cell therapies each year since KYMRIAH® approval in August 2017, the following estimation was used:
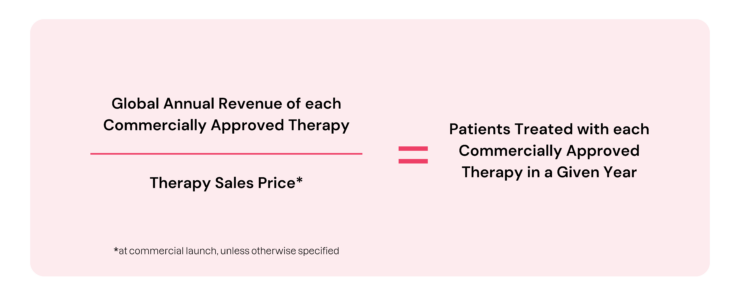
As shown in the table above, the calculation covers patients treated from each therapy’s launch date through 2024. As an additional disclaimer, the patient numbers calculated are intended to be directional in highlighting patient access to these life-saving treatments and not an exact count of the number of patients treated with commercially available therapies.
As an example, recent statements in Gilead’s Q3 2024 earnings presentation note that “more than 25,000 patients treated with Kite’s CAR T-cell therapies to date” (which is assumed to include both clinical and commercial patients).
Other elements the calculations do not account for are a) exact listed sales prices, b) potential discounts on the listed sales prices as the calculation assumes the full list price is charged for every treatment and c) only considers commercial patients and not patients treated outside of a standard commercial setting (e.g. compassionate use, clinical trials, etc.)