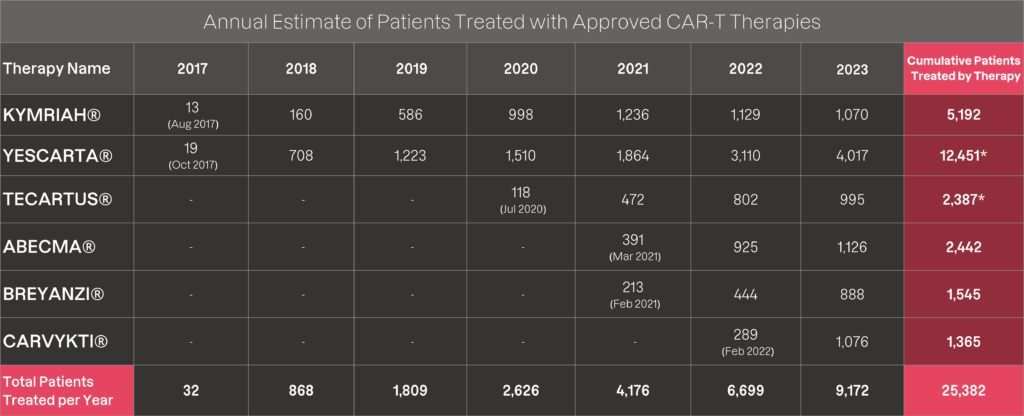
To calculate the number of patients that have had the opportunity to access commercially available
CAR-T therapies each year since KYMRIAH® approval in August 2017, the following estimation was used:
Updated February 2024
* The cumulative data calculated by Ori Biotech for the YESCARTA® and TECARTUS® patient treatments, differs to the statement released by Gilead
To calculate the number of patients that have had the opportunity to access commercially available
CAR-T therapies each year since KYMRIAH® approval in August 2017, the following estimation was used:

As shown in the table above, the calculation covers patients treated with CAR-T from each therapy’s launch date through 2023. As an additional disclaimer, the patient numbers calculated are intended to be directional in highlighting patient access to these life-saving treatments and not an exact count of the number of patients treated with commercially available CAR-Ts.
As an example, Feb 2023 public statements by Gilead indicate “the company has gone from treating a few hundred patients with [YESCARTA® and TECARTUS®] CAR T-cell therapy to now nearly 13,000 globally” with additional data provided in their February 2023 Resource Book. In addition, Kite recently presented at the 2023 ISCT conference in Paris, noting 14,300+ patients treated (clinical and commercial patients).
Other elements the calculations do not account for are a) potential changes to the listed sales prices, b) potential discounts on the listed sales prices as the calculation assumes the full list price is charged for every treatment and c) only considers commercial patients and not patients treated outside of a standard commercial setting (e.g., compassionate use, clinical trials, etc.)
KYMRIAH®
YESCARTA®
TECARTUS®
ABECMA®
BREYANZI®
CARVYKTI®